Electrolyte Polymers ( Polyampholytes )
| Polymers, which are chained molecules with carbons as the backbone, are the materials for engineering products and the building blocks of life. We briefly describe our molecular dynamic simulation studies of randomly charged polymers (polyampholyte), focusing on the structure formation by the electrostatic forces, which model the folding of proteins and the DNA. Charged polymers, sometimes called electrolyte polymers, are roughly divided into two categories, polyelectrolyte, with monomers of the same charge sign, and polyampholyte with randomly arranged monomers of both signs. This simple category works well and represents the typical characteristics of charged polymers. For example, the DNA is strongly and negatively charged polyelectrolyte, with an electronic charge (-e) residing in every nucleic acid of either A, G, C or T. 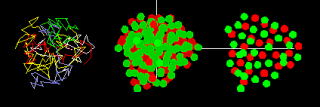 A Coulomb crystal (top) and amorphous A Coulomb crystal (top) and amorphous (bottom), showing inside polymer chains, the positive and negative monomers in the bird's-eye view and the crosscut, from the left to right panels, respectively.  (Phys.Rev.E62, 2000) (Phys.Rev.E62, 2000)In water at room temperature, the electrostatic energy exceeds by several times thermal energy. Polyelectrolyte takes a stretched state due to repulsion among the monomers of like-sign charges, against bending forces between monomers. On the other hand, polyampholyte takes a loose globule because monomers of randomly sequenced charges not only cancel average forces but positive and negative monomers correlate to form pairs. Thus, we can control the softness of charged polymers by changing temperature, pH and salt density. The biochemical processes might be making good use of these properties. |